
Avelumab (Anti-PD-L1) in Relapsed/Refractory Classical Hodgkin Lymphoma
Classical Hodgkin lymphoma (cHL) is characterized by the presence of Hodgkin and Reed-Sternberg tumor cells. In cHL, these malignant cells become surrounded by an immunosuppressive tumor microenvironment composed largely of reactive immune cells. Frequently, amplification of chromosome 9p24.1 in the tumor cells results in overexpression of immune-checkpoint genes that encode programmed death ligand 1 (PD-L1) and PD-L2 proteins. The binding of PD-L1 and PD-L2 on tumor cells and tumor-infiltrating immune cells to the PD1 receptor on T cells deactivates T cell cytotoxic ability. This allows the tumor to evade the immune system. Therefore, new therapeutic strategies that reactivate the antitumor immunity, such as PD-1/PD-L1 blockade, are needed.

Two anti-PD-1 antibodies, nivolumab and pembrolizumab, are currently approved for use in patients with relapsed/refractory (R/R) cHL. These anti-PD-1 antibodies block both the PD-1/PD-L1 interaction and the PD-1/PD-L2 interaction. However, it is unknown if PD-L1 inhibitors, which block only the PD-1/PD-L1 interaction but leave the PD-1/PD-L2 interaction intact, are sufficient to achieve a therapeutic effect in cHL. Avelumab is one such antibody that specifically inhibits PD-1/PD-L1 interactions. The JAVELIN Hodgkins study was a phase 1b, open-label clinical trial to assess the pharmacokinetics, efficacy, and safety of avelumab in patients with R/R cHL.
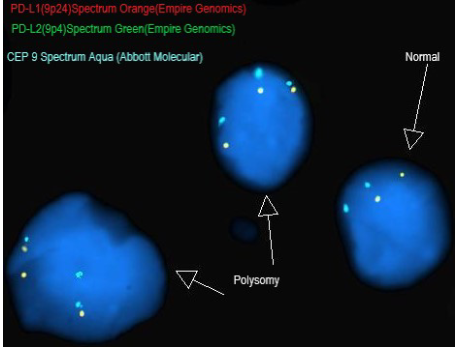
The JAVELIN Hodgkins trial enrolled 31 patients with cHL that either relapsed after a prior autologous hematopoietic stem cell transplant (auto-HSCT) or allogenic HSCT (allo-HSCT) or who were ineligible for HSCT. Patients were randomly assigned to one of 5 avelumab treatment groups with regimens chosen to explore the influence of different doses, dosing frequencies, and fixed vs body weight-based dosing on pharmacokinetics (PK) and the target occupancy (TO) time course. The primary end points were percentage of TO by dose/schedule in peripheral blood immune cells and PK parameters. Secondary end points were safety and antitumor activity. Fluorescence in situ hybridization (FISH) was also performed on a small subset of study participants to determine 9p24.1 alterations of PD-L1 and PD-L2 genes. Of 4 FISH-evaluable samples, two showed PD-L1/PD-L2 copy number alterations.
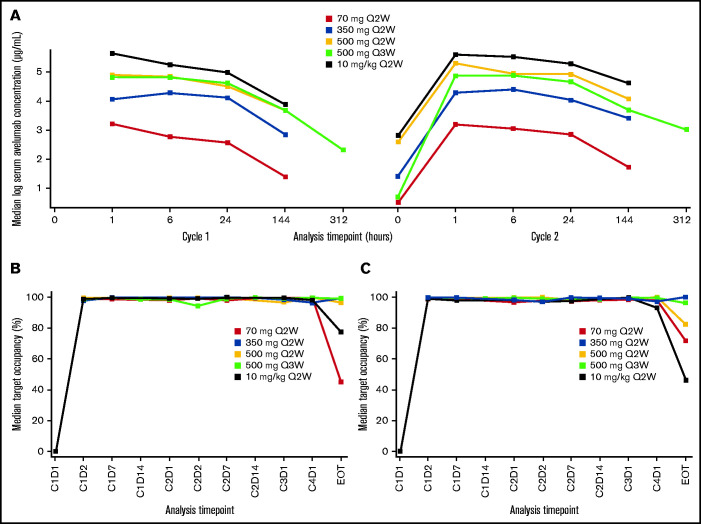
The PK of avelumab was dose-proportional across all dose cohorts with similar PK characteristics for dosing intervals of every 2 weeks and every 3 weeks. Target occupancy (TO) >90% was sustained throughout the dosing interval, regardless of dose level or schedule. Treatment related adverse effects (TRAEs) observed in this study were consistent with the known safety profile of avelumab. The most common TRAEs of any grade were infusion-related reaction, nausea, increased alanine aminotransferase, rash and fatigue. One treatment related death occurred in the avelumab 350-mg every 2 weeks treatment group (pneumonitis), and two patients developed graft vs host disease in the liver. The objective response rate (ORR) was 41.9% for all treatment groups combined and 55.6% in those with a prior allo-HSCT; this was lower than the ORR range observed with nivolumab or pembrolizumab. However, these results suggest that a PD-L1 blockade may be sufficient to produce clinical responses in some patients with heavily pretreated cHL. More studies should examine the role of anti-PD-L1 and anti-PD-L2 in PD-1 blockade therapy. Due to decreased use of allo-HSCT in patients with R/R cHL, the expansion phase of this study was terminated after enrolling three patients.

