Clinical & Research Blog
Cerebrovascular β-Amyloid Deposits Linked to Altered Gene Expression Profile in Mice with Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common form of dementia in older adults. Cerebral amyloid angiopathy (CAA), which is characterized by the deposition of β-amyloid (Aβ) peptides within cerebral blood vessels, co-occurs with AD to some extent in 85-95% of AD cases and is an independent contributor to AD dementia. Indeed, cerebrovascular dysfunction, possibly resulting from Aβ deposits, has been implicated in early AD pathogenesis, preceding neuronal damage. In this study, the authors profile the gene expression patterns of cerebral endothelial cells to identify cerebrovascular pathways that may be involved in the early development of CAA pathology in AD. The cerebral endothelium may play a central role in mediating vascular responses in CAA/AD since it helps to regulate vascular tone and is responsible for Aβ clearance. Thus, profiling the cerebral endothelial transcriptome (i.e., the vasculome) may provide valuable information about the early development of CAA/AD.
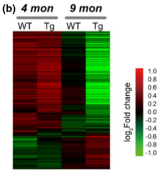
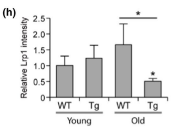
A transgenic mouse line was generated that expressed the Swedish mutation in amyloid precursor protein (APPswe) and a deletion in exon 9 of presenilin 1 (PSEN1). These mutations have been linked to increased Aβ production and early-onset AD, and mice with these mutations begin to develop vascular Aβ mutations at 6 months of age. So, to investigate the early impact of vascular Aβ deposits on the cerebral vasculome, the authors isolated cerebral endothelial cells from 4-month-old transgenic mice (with no obvious Aβ deposits) and from 9-month-old transgenic mice (with mild Aβ deposits). Endothelial cells from age-matched nontransgenic (WT) littermates were used as control. The transcriptome of each group was profiled using microarray. The gene expression patterns of 4-month-old transgenic mice (before Aβ deposits) were like those of age-matched WT mice, but by 9 months old (early disease stage), differential gene expression patterns were observed via heatmap. Notably, low density lipoprotein receptor-related protein 1 (LRP1), which is responsible for mediating Aβ clearance, showed significantly decreased expression in 9-month-old transgenic mice in comparison to WT control mice, indicating impaired Aβ clearance in transgenic mice brains. This result was confirmed by immunohistochemistry staining.
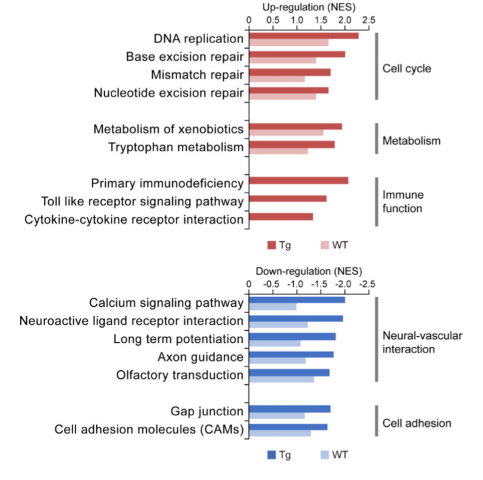
Functional enrichment analysis using GSEA identified that cerebrovascular Aβ deposition in transgenic mice was related with changes in endothelial functions. The authors note that similar functional alterations were observed during aging of the WT mice, but to a lesser extent than that seen in the transgenic mice. This suggests that CAA/AD progression in the vasculome may be related to an accelerated process of aging. Vascular Aβ deposition in transgenic mice was associated with the downregulation of the glutamate and GABA neurotransmitter receptors, as well as decreased calcium signaling. These changes could result in an impaired vascular capacity to sense neural activity and could impact vasodilation, resulting in a deficiency in cerebral blood flow and contributing to early CAA/AD pathology. Further, Aβ accumulation in transgenic mice was associated with decreased expression of cell adhesion molecules, which would disrupt neurovascular coupling; previous studies have linked neurovascular decoupling with the severity of CAA.
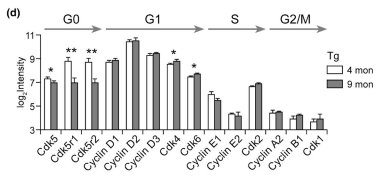
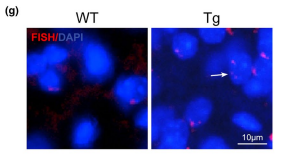
Other functional alterations in the vasculome were noted as well. Analysis revealed that cerebral endothelial cells of transgenic mice showed upregulation of complexes related to DNA synthesis and G1 markers (e.g., Cdk4 and Cdk6) combined with downregulation of G0 markers (e.g., Cdk5, Cdk5r1, Cdk5r2) and low levels of G2/M markers (cyclin A, cyclin B, Cdk1). This suggests erroneous cell cycle reentry in quiescent endothelial cells, and the mismatch between DNA duplication and cell division can generate polyploid cells. Fluorescent in situ hybridization confirmed the presence of polyploidy in the 9-month-old transgenic mice and absence of polyploidy in the 9-month-old WT control mice, suggesting that mice with Aβ deposits may be more prone to genomic instability and added endothelial dysfunction. Finally, vascular Aβ deposits in transgenic mice were associated with upregulation of the toll-like receptor (TLR) signaling pathway and cytokine-cytokine receptor interactions, suggesting that endothelial Aβ accumulation may lead to enhanced inflammation. The authors conclude that, while additional studies should be conducted to validate their findings, endothelial alterations including neurovascular decoupling, erroneous cell cycle reentry, and enhanced inflammation may play a role in early CAA/AD pathogenesis and serve as potential therapeutic targets for these diseases.

Learn more about Alzheimer’s Disease probes here
https://empiregenomics.com/fish-probes/gene/DBF4Citation & Source Link
Deng W, Guo S, van Veluw SJ, Yu Z, Chan SJ, Takase H, Arai K, Ning M, Greenberg SM, Lo EH, Bacskai BJ. Effects of cerebral amyloid angiopathy on the brain vasculome. Aging Cell. 2022 Aug;21(8):e13503. doi: 10.1111/acel.13503. Epub 2022 Jul 18. PMID: 35851991; PMCID: PMC9381891.
https://onlinelibrary.wiley.com/doi/10.1111/acel.13503