Clinical & Research Blog
Amplification of 1q21 Linked to Poor Outcomes of Daratumumab-Based Treatments in Multiple Myeloma
Multiple myeloma (MM) is a genetically heterogeneous disease, and this genetic heterogeneity can contribute to varied clinical responses and survival outcomes among patients. One of the most common cytogenetic abnormalities, occurring in around 40% of newly diagnosed MM cases and up to 70% of relapsed/refractory MM cases, is gain or amplification of chromosome arm 1q21 (1q21+). Recent research has linked 1q21+ to genomic instability, proliferation, drug resistance, and speed of disease progression; these aggressive characteristics result in part from the upregulation of several genes within the 1q21 amplicon, including CKS1B, PSMD4, ANP32E, and MCL-1. Patients with amp1q (≥4 copies of 1q21) generally have a poorer prognosis than patients with gain1q (3 copies of 1q21). Despite the availability of new treatments for MM, including immunomodulatory drugs, proteasome inhibitors, and monoclonal antibodies, effective treatment options for MM with 1q21+ remain elusive. In general, the presence of 1q21+ is linked with shorter durations of progression-free survival (PFS) and overall survival (OS) across treatment type. Daratumumab, an anti-CD38 monoclonal antibody that has recently been licensed for use in relapsed/refractory MM (RRMM) and newly diagnosed MM, has been clearly demonstrated to improve outcomes for MM patients. However, key daratumumab trials did not report outcomes for the 1q21+ patient subgroup, so data regarding its effectiveness in this patient population are scarce. Here, the authors report a retrospective series of 8 RRMM patients with amp1q who received daratumumab-based triplet regimens.
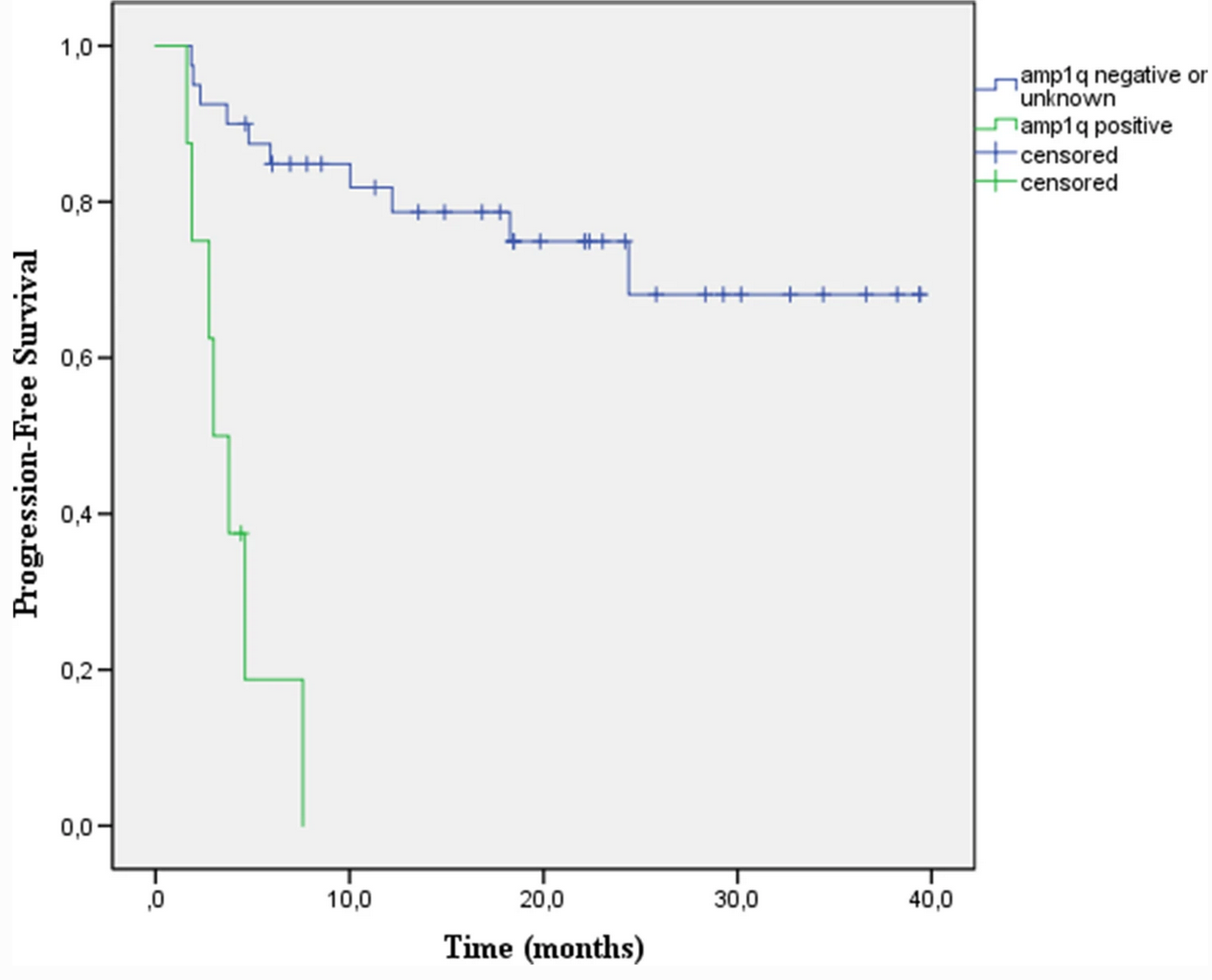
Seven 1q21+ patients received the daratumumab treatment at first relapse, while one 1q21+ patient received daratumumab treatment at second relapse. The presence of 1q21+ was verified using interphase fluorescent in situ hybridization, and all eight patients had ≥4 copies of 1q21. Median follow-up time was 10.1 months (3.0-17.7 months). Of these eight patients, only one achieved very good partial response; 7 patients discontinued treatment due to disease progression, and 4 of these patients have died. Median PFS was 3.0 months (1.6-7.6 months). In contrast, for 40 RRMM patients without amp1q who received daratumumab treatments at comparable disease stages during the same time period, the median PFS was not reached after a median follow-up for 18.4 months (2.3-39.4). These data suggest that 1q21+ patients respond very poorly to daratumumab treatment, and the authors propose that novel treatment options be considered for this MM patient subpopulation.
