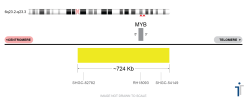
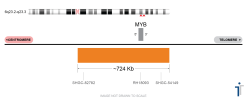
Melanoma
Concentrate
Price range: $1,670.00 through $3,172.00
Dual Color
Price range: $1,161.00 through $2,354.00
Concentrate
Price range: $955.00 through $1,931.00
Concentrate
Price range: $955.00 through $1,692.00
Chromosome
$390.00
CE-IVD
Price range: $526.00 through $1,692.00